 Hire a Tutor
Hire a Tutor 




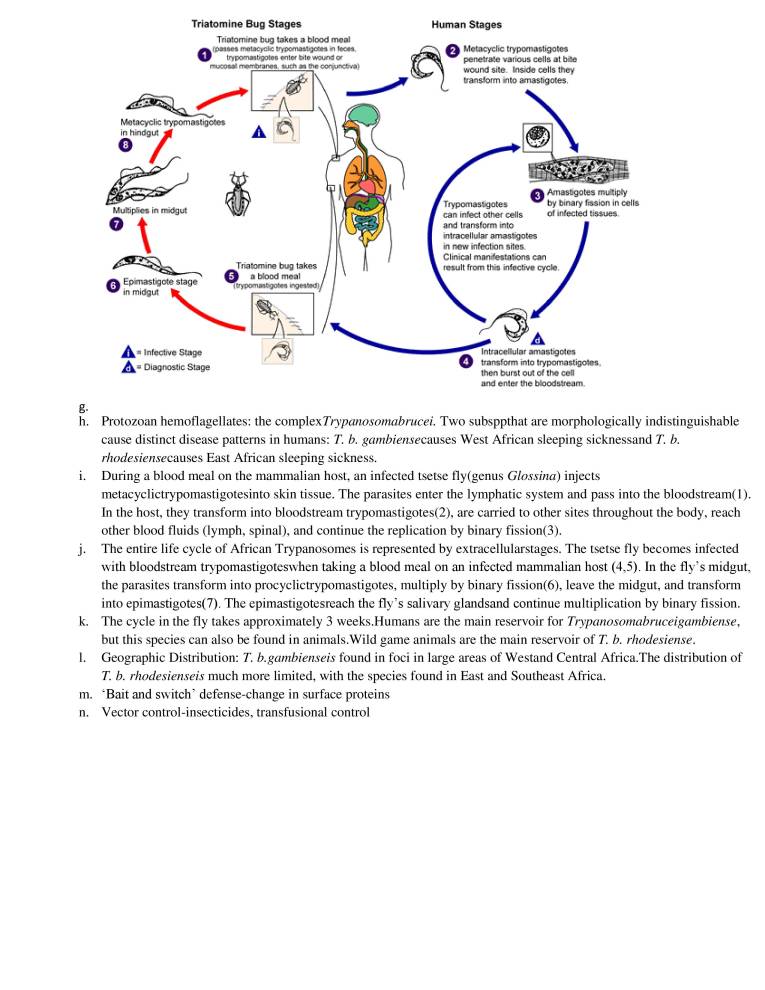



Point form with illustration and diagram make students easy to understand the concept.
10 years of teaching experience
Qualification: Master
Teaches: English, Chinese Mandarin, Accounts, Mandarin, Mathematics, Additional Math, Modern Maths, Flute, Keyboard, Cello, Music Theory, Biology, Accounting, Additional Maths, Math
